- Open-Access Publishing
- Quality and Potential Expertise
- Flexible Online Submission
- Affordable Publication Charges
- Expertise Editorial Board Members
- 3 Week Fast-track Peer Review
- Global Visibility of Published Articles
Age Role in the Clinical Management of Urological Cancers
Nazario Foschi*, Marco Campetella*, Luca Di Gianfrancesco, Mauro Ragonese, Pierluigi Russo, Pierfrancesco Bassi
Department of Urology, Catholic University of Sacred Heart, Policlinico A. Gemelli, Rome, Italy
*Corresponding Author:
*Marco Campetella, Department of Urology, Catholic University of Sacred Heart, Policlinico A. Gemelli, Rome, Italy, E-mail: marco.campetella@yahoo.it
Nazario Foschi, Department of Urology, Catholic University of Sacred Heart, Policlinico A. Gemelli, Rome, Italy, E-mail: nazariofoschi@yahoo.it
Received date: June 03, 2021; Accepted date: July 14, 2021; Published date: July 21, 2021
Citation: Foschi N, Campetella M, Di Gianfrancesco L, Ragonese M, Russo P, et al. (2021) Age Role in the Clinical Management of Urological Cancers. Arch Neph Urol Stud. 1:01.
Copyright: © 2021 Foschi N. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Keywords
Age; Cancer; Urology; Surgery
Abstract
Urological cancer incidence is increasing worldwide and these cancers represent a big challenge for both patients and physicians. Age is not only a risk factor for these cancers, but also represents a very important feature to be evaluated for prognosis and in every decision-making process, playing a crucial role for these patient’s management. As most aggressive tumor occur in older patients, there are many other aspects to be considered in this population to find out the best treatment option. Cancers in younger patients represent also a huge challenge for clinicians, with very specific features, in particular about renal cancer. In addition to this, over and under treatment of specific urological tumors can occur, with age being a key factor leading to different treatment options. Other patient’s factors, like comorbidities and life expectancy also play an important role in this setting an must be given full consideration.
With this paper, we wanted to underline the importance of age for the therapeutic decisions and prognosis of patients affected by renal cell cancer, bladder cancer, upper urothelial tract cancer and prostate cancer.
Introduction
Urologic cancer burden has increased globally amid population growth and ageing. Each year, about 2 million renal (RCC), bladder (BC) and prostate cancer (PCa) cases occurred worldwide, increasing 2.5-fold since 1990. The majority of new cases in 2013 occurred in individuals aged 60 years old or older [1]. Until 60 years ago, children under 5 years of age were numerically more than twice than people aged over 65. In the last 60 years, the trend has completely changed, reversing this paradigm. Population ageing is expected also to increase even more in the next 50 years, due to the drop in fertility rates and the increases in life expectancy (Figure 1).
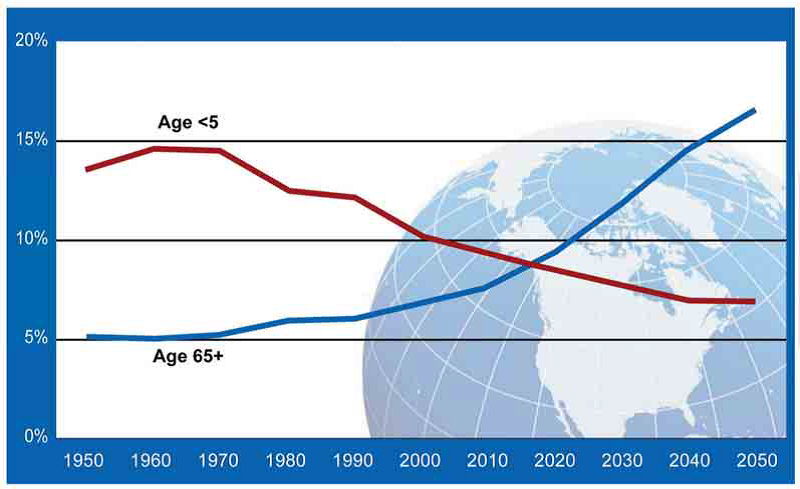
Figure 1: United Nations. World population prospects: the 2010 revision. Red line: people under 5 years of age; Blue line: people over 65 years of age; X-axis: date; Y-axis: percentage of general population.
Age represents not only a risk factor but a variable to keep in consideration in the screening or in the diagnostic phases, and also in the developing of the most appropriate management, even if it is not always considered in prognostic models or nomograms that are routinely used. Not only chronological age but also comorbidities and Performance Status (PS) should be considered in planning any kind of diagnostic or therapeutic procedure [2].
Under a biological point of view, the same cellular dysfunctional mechanisms characterizing the aging process, such as accumulation of genetic and epigenetic changes, the diminishing of telomere length, the progressive disruption of mechanisms for DNA damage repair, loss of glucose metabolism regulation and cell cycle control, have been found to be involved, with different level of evidence, in carcinogenesis [3].
As the life expectancy grows, it becomes more evident that older persons bear the majority of cancer burden; on the other hand, the count of comorbid conditions increases with advancing age among general oncology patients [4].
Patient-related factors like age, Charlson Comorbidity Index (CCI) or Eastern Cooperative Oncology Group (ECOG) score should be the more significant and reliable contributors to decision-making process predicting Overall Survival (OS) for most malignancies, including PCa, RCC and BC [5-7].
To date, correct life expectancy estimation and competing risk evaluation is not well characterized with an increasing quote of frail patients exposed to the risk of overtreatment and another quote of healthy aged people exposed to the undertreatment risk.
The aim of this narrative review is to underline the role of age in the more common urological malignancies, its prognostic significance and its weight on the therapeutic choices.
Materials and Methods
Literature review was performed in January-February 2020, no temporal limits were adopted. Studies were identified on Pubmed, Scopus and Web of Science with the following keywords: “age”, “lifetime risk”, “age of onset”, “competing risk”, and “renal cell cancer”, “bladder cancer”, “upper tract urothelial cancer”, “prostate cancer”. All the retrieved items were screened with the purpose to identify papers reporting on age role in urological diseases. The research was extended to any pertinent issue on this topic.
Discussion
Renal cancer
Kidney cancer is the ninth most commonly occurring cancer in men and the 14th most commonly occurring cancer in women. There were over 400,000 new cases in 2018. The average age at diagnosis is 64 years old. The gold standard therapy for renal cancer is radical surgery.
There are various prognostic factors in RCC, but the most popular prognostic models for prediction of Cancer Specific Mortality (CSM) do not include age [8-10]. Recently, a preoperative prognostic model based on age, symptoms, and TNM staging (Tumor-Nodes-Metastasis) has been validated from big multicentre international database, and age results highly statistically significant predictor of Cancer Specific Survival (CSS) at univariate analysis [11]. In renal cancer, survival is very high (up to 90% of 5-y CSS) in Stage I and II disease (T1 or T2), while it drops to about 64% in stage III disease (T3 or N1) and to 11% in stage IV disease (T4 or M1) [12].
Age in RCC was investigated in different topics. It was supposed that RCC arising in young adults could be more symptomatic and potentially aggressive; but in the elderly population there is an increasing number of tumours detected incidentally, hypothesizing a potential indolent behaviour for their typical slow growth rate. The results of historical series addressing these issues are not conclusive, some Authors didn’t recognize changes in prognosis for different ages of onset, with poor variability about histopathological subtype, nodal status or presence of metastasis at presentation [13,14]. Gillet et al reported better but not significantly different 5 and 10 years CSS for younger versus older RCC patient [15]. Sanchez-Ortiz et al observed less advanced stages at the diagnosis but a surprisingly more frequent lymphnode involvement in the young population group who, however, had a better survival than the older group [16].
In literature, early onset RCC is described in 4-7.5% of adults under 40 years old, and many case series seem to confirm less aggressive cancers with a lower stage and grade at presentation and a better prognosis. Verhoest et al reported about 300 early onset RCC and analysed difference in clinical presentation and survival between younger patients group (under 40 years old) and older groups. They described a better CSS for the early onset RCC with a lower tumour stage and grade, and a more balanced male/female ratio in this group [17].
In a large French multicentre database, Authors reported better CSS and Progression Free Survival (PFS), lower stage and lower male:female ratio in population under 40: differently from the previous cited evidences, the proportion of papillary RCC (pRCC) was higher than clear cell RCC (ccRCC) and chromophobe RCC (chRCC) [18]. Also in the database by Verohest et al, both chRCC and pRCC were more frequent in the early onset cancers, ccRCC became more frequent in the older population [17].
Denzinger et al investigated the different clinical pathological features of two series of RCC, under 45 and over 75 years old, describing lower stage and grade in the younger group, with chRCC more frequent in the older group; young age emerged as independent predictor of better survival [19].
Cai et al found 45 years of age was the optimal cut-off point that maximizes the predictive value of age on the CSS and confirmed on multivariate competing risk regression analysis the age under 45 years old as an independent prognostic factor for better CSS [20].
In the Small Renal Masses (SRMs) subset, similar results indicate a better Recurrence Free Survival (RFS) after surgery in the young patients (<40 years old) with a lower grade and a lower stage at presentation [21]. In this setting, Active Surveillance (AS) is based on the evidence of the lower RCC specific mortality in elderly and frail population, because of significant competing Other Cause Mortality (OCM).
There is also an important impact on Quality of Life (QoL): in elderly population, active treatment, even if invasive, led to a physical health benefit, while AS could led to depression or anxiety [22]. However, Sun et al reported higher Cancer Specific Mortality (CSM) rates in more advanced patient age with more detrimental effect on the stage I disease, being unexpectedly less evident for stages II-IV [11,23].
Thus, a possible undertreatment has to be considered in this population, in which the potential for curative disease management should not be denied; on the other hand, the risk of overtreatment is to be considered much more relying on comorbidities or performance status instead of just age.
Why renal tumours in elderly seem more aggressive still remains unclear, a hypothetical reason could be a better tumour control in the young people owing a superior immunological response or better immunological tumour-host response than the older and frail population in which “immune-senescence” could allow a more aggressive tumour development [19,21].
Metastatic RCC (mRCC) is a completely different clinical situation, where several prognostic factors are recognized and used for mRCC, and most predictive models like the Memorian Sloan Kettering Cancer Centre (MSKCC) don’t include age. However, some evidences support age as a prognostic factor in this patient’s subset. Song et al found that age and BMI were independent adverse prognostic factors in mRCC: median survival of patient aged <45 years old was half than the >45 years old [24]. Zhang et al found people <65 years old receiving sorafenib or sunitinib as first line therapy in mRCC had shorter PFS and shorter OS than elderly patients [25]. Conversely, Pal et al found lower median survival in elderly patients than in the younger (12.5 vs. 26.4 months), as the firsts seem more prone to discontinue therapy. Probably, elderly in many cases receive few lines of systemic therapies and discontinue them for comorbidity or toxicity [26].
Bladder cancer
Bladder cancer is the sixth most commonly occurring cancer in men and the 17th most commonly occurring cancer in women. There were almost 550,000 new cases in 2018. The average age of people when they are diagnosed is 73. BC is primarily considered a disease of the middle-aged and elderly with a typical high male/female ratio. The increasing age is now widely accepted as a strong and independent risk factor of BC, and the two most important prognostic scores (EORTC and CUETO) include age at diagnosis.
The prognosis between the younger patients and their elderly counterparts is controversial, as well as the definition of young BC patients. Some studies defined the young BC patients as less than 40 years, while in other studies a threshold cut off age 70 also was used [27-29].
Even if rare, BC could present also in paediatric age and in adolescence with a better clinical outcome and a lower rate of disease recurrence, at least until the 19 years old, after this cut-off BC behaviour replicates the adult one [30]. The better survival of younger patients is explained by the better overall function, faster postoperative recovery and the higher tolerance to more aggressive and toxic therapies. These issues are challenging in the management of both non-muscle invasive BC (NMIBC) and muscle invasive BC (MIBC).
In NMIBC, patient’s age could influence the response to intravesical therapies, conditioning prognostic outcomes like tumour recurrence or progression. BCG (Bacillus Calmette-Guérin) therapy requires a competent immune system, the elderly people may not respond adequately to the treatment and moreover be exposed to more serious side effects. Despite guidelines recommendations, only 25% of eligible patients undergoes BCG immunotherapy: very often frail, comorbid and elderly patients are undertreated because are considered at high risk of serious complications [31]. Herr et al reported no statistically significant differences in terms of initial response to BCG therapy between younger and older patients, but at five years 37% of patients younger than 70 years old were disease-free compared to 27% of older patients [32]. Investigating age influence on BCG response, Yuge et al evaluated 447 non-invasive high grade BC and although they didn’t find age to be an independent predictor of tumour recurrence they showed that patient 55-64 years old were continuously tumour-free than patients aged 75 or more [33]. Conversely Mulders et al found age having no association with prognosis in a multivariate analysis even if the age cut off was set at 65 years [34]. BCG serious complications are well described in the elderly, but a clear relationship with age is not proven [35].
Heiner et al reported an association between age and side effects of intravesical BCG with about three-fold higher rate of complications in the group over 70 years old; the elderly had also worse response to therapy [36].
In MIBC, the critical point is the possible necessity of a major surgical procedure like Radical Cystectomy (RC), its related risks should be weighted on the real survival benefit and on symptoms relief, particularly in elderly population. So, age again represents one of the key factors that might move the surgeon between whether to operate the patient or not. Young patients surely show the best profile of tolerability and the lowest risk class for surgery bearing better oncologic outcomes. Feng et al reported young patients under 50 with BC appear to have a higher CSS after surgery, compared to the elderly [37]. Resorlu et al reviewed about 250 BC patients who underwent RC finding a significant association between age and more advanced stage, with adverse clinical and pathological features and poorer cancer specific outcomes [27].
Historical series didn’t show age to be an independent predictor of survival, but more recent series found an increased risk of extravescical disease, disease recurrence, poorer cancer specific outcomes and survival for older patients, as reported by Nielsen et al [27,38,39]. Koppie et al determined the association between age and comorbidity on OS and CSS after RC on about 1120 patients, showing CSS seems similar between older and younger patients [40]. Novotny et al investigated the efficacy of the most common scores and classification together with age to estimate the 90 days perioperative risk in about 1000 BC patient undergoing RC. In addition to age and CCI, they found ASA (American Society of Anesthesiology) classification as a suitable classification in patients selected for RC; all of these were independent predictors of perioperative mortality [41]. Weizer et al reported that PS was an independent predictor of OS on patients aged ≥ 70 years presenting with non-metastatic MIBC, confirming also that in many elderly patients with a good PS, radical surgery offers the best disease control [42]. Increased risk of advanced stage and poor CSS among the elderly could also be due to a generalized reluctance to treat these patients with major surgery causing a delay in treatment decision, particularly in smaller and low volume centres [43].
Upper urinary tract carcinoma (UTUC)
UTUC has an estimated annual incidence of 1-2 cases per 100,000, and the mean age at diagnosis increased over the last three decades from 68 to 73 years [44].
UTUC is a rare disease and it is challenging to generate high level evidences regarding prognostic factors and risk stratification. TNM staging is similar to bladder cancer, including subepithelial connective tissue invasion (T1), muscular invasion (T2), peripelvic/periureteric fat invasion (T3) and other organs invasion (T4); lymph node invasion is another big negative prognostic factor. Moreover, UTUC still represent an aggressive malignancy with high recurrence and progression rate. Rouprêt et al found that age was not associated to intravesical recurrence while was significantly associated to PFS, CSS and to OS [45]. Conversely, Elenkov and al failed to find significant relationship with age stressing role of pathological factors and mainly lymphovascular invasion [46].
Krabbe et al reported a classification accuracy of 72.8% for their optimized nomogram including age, stage, grade and tumour architecture. They identified 4 risk groups (low, intermediate, high and very high risk) with the highest relapse rate observed in lower age at RNU with higher pT/pN stages and sessile tumour architecture [47].
Shariat et al evaluated how age affects the indications and outcomes of radical nephroureterectomy (RNU) as the effectiveness and relative use of RNU in the subgroup of older patients remain controversial [48].
Elderly patients were less likely to undergo lymphadenectomy or receive postoperative chemotherapy for advanced stage disease as similarly previously reported for BC [40,49].
They concluded that being older at the time of RNU was associated to a decrease in survival hypothesizing a potential change in tumour biological behaviour and an impaired immunological defence mechanism; interestingly the rate of high stage tumor increased with age. Alternatively, a suboptimal use of RNU or lymphadenectomy or adjuvant chemotherapy can lead to an impaired effect on survival for elderly group [48].
Prostate Cancer (PCa)
PCa is the second most commonly occurring cancer in men (but the most common in men aged 70 or more) and the fourth most commonly occurring cancer overall. There were 1.3 million new cases in 2018, and PCa is considered one of the most exemplifying case of strict relationship between malignancy and age. Age represent one of the best recognized risk factor for PCa but the independent impact of age on PCa specific survival has not been well established, however, age has been included in most prognostic nomograms. There are controversial data regarding the relationship between age of onset and PCa behaviour; in several series, younger age of onset has been correlated with more aggressive tumours and higher CSM, in other reports survival rates seem to be equivalent or superior in younger patients. Other studies describe a higher incidence of higher grades and advanced stage of presentation in the elderly. The importance of age in PCa biological features remains unclear; but it seems incorrect to advocate that elderly patients do not need curative treatments basing on the misperception that these tumours may be less aggressive [4,50].
PCa is treated with different approaches, like radical prostatectomy, radiotherapy, ormonal therapy and chemotherapy, depending on grade and stage of the cancer, being the first two the best approaches for localized disease. In this setting, age might be strongly considered when deciding to perform surgery or not, having radical prostatectomy side effects that are heavier in elderly people.
Age and PSA value are considered both main characters in the evaluation of PCa risk and were gathered in many nomograms created to increase PSA performances [51,52].
In young males, evaluating PSA for early PCa detection might be offered in a risk adapted strategy, but overdiagnosis and overtreatment still represent two big issues. Despite recommendations discouraging PSA based screening, a significant proportion of men with limited life expectancy continues to be screened for PCa [53,54]. In these men, OCM must be accurately examined, cardiovascular morbidity is the main factor to be considered as it represents a consistent risk of mortality in PCa patients but on the other hand, life expectancy continues to increase [55,56].
To date, many insignificant lesions do not require any active treatment: in elderly, the strategies should be balanced between harms and benefits avoiding overtreatment and overdiagnosis in a comorbid and frail population. Nevertheless, a 71% of PCa related deaths occurs in men aged >75 years in the Surveillance, Epidemiology and End Result Program (SEER) database with a higher incidence of advanced and metastatic disease. Despite this, the risk of undertreatment is a concern in western countries; and 41% of men aged >75 years old received curative treatment for intermediate and high risk PCa.
MacKintosh et al recently examined the Veteran Affairs database on 230000 men aged 50-89 years to evaluate the relation between PSA and age prior to PCa diagnosis and 10-year CSM. They calculated CSM for each combination of age and PSA value, and observed that 1.2 years of age had approximately the same influence of 1.0 unit of PSA. PSA and age were both strong independent predictors of PCa specific death and were more predictive in combination, underlining the risk of underestimating the potential harms of PCa in the elderly population overrating the competing risk mortality [57].
Despite the increased prevalence in the elderly, recommendations against screening in men older than 75 are based on studies on younger patients irrespective of comorbidities and health status. Reports have found a high prevalence of both undertreatment and overtreatment in elderly patients, so age represents a source of disparity in treatment offer and clinical trial recruitment too [58-61]. Many evidences do support the use of surgery or radiotherapy in carefully selected patients older than 75 or 80 years old with high risk diseases [62,63].
However, a big volume of literature stressed the opposite point of view in which a lot of men with limited life expectancies are unlikely to benefit from PCa screening, biopsy, early disease detection and finally therapy. Many other basic factors should be considered, particularly comorbidity and PCa class risk to give the more accurate prognostic information. Bechis et al confirmed the increased likelihood of high risk PCa in elderly, and a substantial variation in treatment based on age more than PCa risk class with a sensible reduction of active treatments in this group. In the group of elderly patients receiving active treatments mortality was observed compared impressive 50% reduction in risk-adjusted mortality compared with same age cohort receiving watchful waiting or antiandrogen therapy; so the underuse of active treatment modalities in these patients explain the higher CSM [64].
From another point of view, in the elderly the effect of therapies and the weight of CSM could be reduced by their reduced life expectancy and the impaired tolerance to cancer related treatments effects. Briganti et al considered about 6000 patients undergoing radical prostatectomy and pelvic node dissection for high risk PCa, stratifying four age classes using 5 years intervals and reporting individual CCI. Older age and presence of comorbidities were significantly associated with OCM. The younger group with multiple risk factors and no comorbidities represent the group with higher CSM, but similarly among older patients with no comorbidities CSM had a comparable impact. However, OCM represented the leading cause of death in all age groups except for only younger individuals (≤ 59 years). Increasing age and CCI were associated with increasing OCM rates irrespective of PCa risk factors [56].
Recently Lunardi et al. focused on the influence of age and comorbidities on PCa active management and the risk of over and undertreatment. They randomized 600 patients stratifying them per PCa risk classes and CCI and defining over and undertreatment using a threshold of 10 years’ life expectancy [65]. They found about 25% of patients were in a situation of potential overtreatment, with 36 young patients undergoing active treatment while they were suitable to AS. Conversely, a 37.8% undertreatment rate was observed among patients who underwent non-curative approaches. The bigger concern was on the latter, as age more than CCI drove treatment decision emphasizing that comorbidity status is not properly considered in life expectancy assessment. Effectively, chronological age still represent the main decisive factor, not considering real individual health status overestimating life expectancy in comorbid patients and underestimating it in older patients without significant associated morbidities. To date, there is still no generally accepted tool for predicting mortality risk in PCa patients.
Conclusion
For all urological malignancies, age represents a key point from a prognostic point of view and is a main factor to be considered in all therapeutic decision-making processes. Age seems to have conflicting significance, probably a protective role is present in younger patients, where most malignancies seem to show less aggressive behaviour. Also, possible delays in the surgical management, a reluctance from the clinicians to apply invasive management with relative risk in a frail population, and a more aggressive cancer biology may be the reason that can explain worse prognosis in the older population.
Comorbidity status is very important too, as it allows a better evaluation of risks in the perioperative period and provides more exhaustive information on oncologic outcomes.
Multiple biases may be due to the retrospective nature of almost all studies, and to big variability in the consideration of life expectancy and lifetable application too. These parameters could help more than chronological age in the decision-making process of all the malignancies we considered, in which the estimation of OCM and competitive risk mortality should be mandatory.
Age, comorbid illness and lifetables must be integrated in treatment decisions and should be considered in researches in a rational and quantifiable way, without forgetting the need of a tailored approach in more controversial cases.
References
01. Dy GW, Gore JL, Forouzanfar MH, Naghavi M, Fitzmaurice C, et al. Global burden of urologic cancers, 1990-2013. Eur Urol. 71(3): 437-446 (2017).
02. Coebergh JW, Janssen-Heijnen ML, Post PN, Razenberg PP. Serious co-morbidity among unselected cancer patients newly diagnosed in the south-eastern part of The Netherlands in 1993-1996. J Clin Epidemiol. 52(12): 1131-6 (1999).
03. Bassi PF, Sacco E. Cancer and aging: The molecular pathways. Urol Oncol. 27(6): 620-7 (2009).
04. Hall WH, Jani AB, Ryu JK, Narayan S, Vijayakumar S. The impact of age and comorbidity on survival outcomes and treatment patterns in prostate cancer. Prostate Cancer Prostatic Dis. 8(1): 22-30 (2005).
05. Gettman MT, Boelter CW, Cheville JC, Zincke H, Bryant SC, et al. Charlson co-morbidity index as a predictor of outcome after surgery for renal cell carcinoma with renal vein, vena cava or right atrium extension. J Urol. 169(4):1282-1286 (2003).
06. Albertsen PC, Fryback DG, Storer BE, Kolon TF, Fine J. The impact of co-morbidity on life expectancy among men with localized prostate cancer. J Urol. 156(1):127-132 (1996).
07. Miller DC, Taub, DA, Dunn RL, Montie JE, Wei JT. The impact of co-morbid disease on cancer control and survival following radical cystectomy. J Urol. 169(1):105-109 (2003).
08. Thompson RH, Leibovich BC, Lohse CM, Cheville JC, Zincke H. Dynamic outcome prediction in patients with clear cell renal cell carcinoma treated with radical nephrectomy: The D-SSIGN score. J Urol. 177(2): 477-80 (2007).
09. Zisman A, Pantuck AJ, Wieder J, Chao DH, Dorey F, et al. Risk group assessment and clinical outcome algorithm to predict the natural history of patients with surgically resected renal cell carcinoma. J Clin Oncol. 20(23): 4559-66 (2002).
10. Kattan MW, Reuter V, Motzer RJ, Katz J, Russo P. A postoperative prognostic nomogram for renal cell carcinoma. J Urol. 166(1): 63-7 (2001).
11. Karakiewicz PI, Suardi N, Capitanio U, Jeldres C, Ficarra V, et al. A preoperative prognostic model for patients treated with nephrectomy for renal cell carcinoma. Eur Urol. 55(2): 287-295 (2009).
12. European association of urology guidelines, renal cell carcinoma. (2020).
13. Aziz A, May M, Zigeuner R, Pichler M, Chromecki T, et al. Do young patients with renal cell carcinoma feature a distinct outcome after surgery? A comparative analysis of patient age based on the multinational CORONA Database. J Urol. 191(2): 310-315 (2014).
14. Thompson RH, Ordonez MA, Iasonos A, Secin FP, Guillonneau B, et al. Renal cell carcinoma in young and old patients: Is there a difference? J Urol. 80(4): 1262-1266 (2008).
15. Gillett MD, Cheville JC, Karnes RJ, Lohse CM, Kwon ED, et al. Comparison of presentation and outcome for patients 18 to 40 and 60 to 70 years old with solid renal masses. J Urol. 173(6): 1893-6 (2005).
16. Sanchez-Ortiz RF, Rosser CJ, Madsen LT, Swanson DA, Wood CG. Young age is an independent prognostic factor for survival of sporadic renal cell carcinoma. J Urol. 171(6): 2160-5 (2004).
17. Verhoest G, Veillard D, Guille F, De La Taille A, Salomon L, et al. Relationship between age at diagnosis and clinicopathologic features of renal cell carcinoma. Eur Urol. 51(5): 1298-1305 (2007).
18. Taccoen X, Valeri A, Descotes JL, Morin V, Stindel E, et al. Renal cell carcinoma in adults 40 years old or less: Young age is an independent prognostic factor for cancer-specific survival. Eur Urol. 51(4): 980-987 (2007).
19. Denzinger S, Otto W, Burger M, Hammerschmied C, Junker K, et al. Sporadic renal cell carcinoma in young and elderly patients: are there different clinicopathological features and disease specific survival rates? World J Surg Oncol. 5: 16 (2007).
20. Cai M, Wei J, Zhang Z, Zhao H, Qiu Y, et al. Impact of age on the cancer-specific survival of patients with localized renal cell carcinoma: Martingale residual and competing risks analysis. PLoS One. 7(10): e48489 (2012).
21. Jeong IG, Yoo CH, Song K, Park J, Cho YM, et al. Age at diagnosis is an independent predictor of small renal cell carcinoma recurrence-free survival. J Urol. 182(2): 445-450 (2009).
22. Pierorazio P, McKiernan J, Allaf M. Quality of life on active surveillance for small masses versus immediate intervention: Interim analysis of the DISSRM (delayed intervention and surveillance for small renal masses) registry. J Urol. 189(4S): 259 (2013).
23. Sun M, Abdollah F, Bianchi M, Trinh QD, Jeldres C, et al. A stage-for-stage and grade-for-grade analysis of cancer-specific mortality rates in renal cell carcinoma according to age: A competing-risks regression analysis. Eur Urol. 60(6): 1152-1159 (2011).
24. Song Y, Du C, Zhang W, Sun Y, Yang L, et al. Body mass index and age are additional prognostic factors in patients with metastatic renal cell carcinoma treated with tyrosine kinase inhibitors. Urol Oncol. 34(6): 258.e15-22 (2016).
25. Zhang G, Zhu Y, Dong D, Gu W, Zhang H, et al. Clinical outcome of advanced and metastatic renal cell carcinoma treated with targeted therapy: Is there a difference between young and old patients? Onco Targets Ther. 7: 2043-52 (2014).
26. Pal SK, Hsu J, Hsu S, Hu J, Bergerot P, et al. Impact of age on treatment trends and clinical outcome in patients with metastatic renal cell carcinoma. J Geriatr Oncol. 4(2): 128-133 (2013).
27. Resorlu B, Beduk Y, Baltaci S, Ergun G, Talas H. The prognostic significance of advanced age in patients with bladder cancer treated with radical cystectomy. BJU Int. 103(4): 480-483 (2009).
28. Linn JF, Sesterhenn I, Mostofi FK, Schoenberg M. The molecular characteristics of bladder cancer in young patients. J Urol. 159(5): 1493-1496 (1998).
29. Fitzpatrick JM, Reda M. Bladder carcinoma in patients 40 years old or less. J Urol. 135(1): 53-54 (1986).
30. Caione P, Patruno G, Pagliarulo V, Bulotta AL, Salerno A, et al. Nonmuscular invasive urothelial carcinoma of the bladder in paediatric and young adult patients: Age-related outcomes. Urology. 99: 215-220 (2017).
31. Spencer BA, McBride RB, Hershman DL, Buono D, Herr HW, et al. Adjuvant intravesical bacillus calmette-guérin therapy and survival among elderly patients with non-muscle-invasive bladder cancer. J Oncol Pract. 9(2): 92-8 (2013).
32. Herr HW. Age and outcome of superficial bladder cancer treated with bacille Calmette-Guérin therapy. Urology. 70(1): 65-8 (2007).
33. Yuge K, Kikuchi E, Matsumoto K, Takeda T, Miyajima A, et al. Could patient age influence tumour recurrence rate in non-muscle-invasive bladder cancer patients treated with BCG immunotherapy? Jpn J Clin Oncol. 41(4): 565-70 (2011).
34. Mulders PF, Meyden AP, Doesburg WH, Oosterhof GO, Debruyne FM. Prognostic factors in pTa-pT1 superficial bladder tumours treated with intravesical instillations. The Dutch South-Eastern Urological Collaborative Group. Br J Urol. 73(4): 403-8 (1994).
35. Huang TC. Management of complications of bacillus Calmette-Guérin immunotherapy in the treatment of bladder cancer. Ann Pharmacother. 34(4): 529-32 (2000).
36. Heiner JG, Terris MK. Effect of advanced age on the development of complications from intravesical bacillus Calmette-Guérin therapy. Urol Oncol. 26(2): 137-40 (2008).
37. Feng H, Zhang W, Li J, Li X. Different patterns in the prognostic value of age for bladder cancer-specific survival depending on tumor stages. Am J Cancer Res. 5(6): 2090-2097 (2015).
38. Clark PE, Stein JP, Groshen SG, Cai J, Miranda G, et al. Radical cystectomy in the elderly: Comparison of survival between younger and older patients. Cancer. 103(3): 546-52 (2005).
39. Nielsen ME, Shariat SF, Karakiewicz PI, Lotan Y, Rogers CG, et al. Bladder Cancer Research Consortium (BCRC). Advanced age is associated with poorer bladder cancer-specific survival in patients treated with radical cystectomy. Eur Urol. 51(3): 699-706 (2007).
40. Koppie TM, Serio AM, Vickers AJ, Vora K, Dalbagni G, et al. Age-adjusted Charlson comorbidity score is associated with treatment decisions and clinical outcomes for patients undergoing radical cystectomy for bladder cancer. Cancer. 112(11): 2384-92 (2008).
41. Novotny V, Froehner M, Koch R, Zastrow S, Heberling U. Age, American Society of Anesthesiologists physical status classification and Charlson score are independent predictors of 90-day mortality after radical cystectomy. World J Urol. 34(8): 1123-9 (2016).
42. Weizer AZ, Joshi D, Daignault S, Kinnaman M, Hussain M, et al. Performance status is a predictor of overall survival of elderly patients with muscle invasive bladder cancer. J Urol. 177(4): 1287-93 (2007).
43. Fahmy N, Aprikian A, Al-Otaibi M, Tanguay S, Steinberg J, et al. Impact of treatment delay in patients with bladder cancer managed with partial cystectomy in Quebec: A population-based study. Can Urol Assoc J. 3(2): 131-5 (2009).
44. Soria F, Shariat SF, Lerner SP. Epidemiology, diagnosis, preoperative evaluation and prognostic assessment of Upper-Tract Urothelial Carcinoma (UTUC). World J Urol. 35(3): 379-387 (2017).
45. Rouprêt M, Babjuk M, Compérat E, Zigeuner R, Sylvester RJ, et al. European association of urology guidelines on upper urinary tract urothelial cell carcinoma: 2015 update. Eur Urol. 68(5): 868-79 (2015).
46. Elenkov AA, Timev A, Dimitrov P, Vasilev V, Krastanov A, et al. Clinicopathological prognostic factors for upper tract urothelial carcinoma. Cent European J Urol. 69(1): 57-62 (2016).
47. Krabbe LM, Eminaga O, Shariat SF, Hutchinson RC, Lotan Y, et al. Post-Operative nomogram for relapse-free survival in patients with high-grade upper tract urothelial carcinoma. J Urol. 197(3): 580-589 (2017).
48. Shariat SF, Godoy G, Lotan Y, Droller M, Karakiewicz PI, et al. Advanced patient age is associated with inferior cancer-specific survival after radical nephroureterectomy. BJU Int. 105(12): 1672-7 (2010).
49. Nielsen ME, Shariat SF, Karakiewicz PI, Lotan Y, Rogers CG, et al. Advanced age is associated with poorer bladder cancer-specific survival in patients treated with radical cystectomy. Eur Uro. 51(3): 699-708 (2007).
50. Bratt O, Folkvaljon Y, Eriksson MH, Akre O, Carlsson S, et al. Undertreatment of men in their seventies with high-risk non-metastatic prostate cancer. Eur Urol. 68(1): 53-58 (2015).
51. van Vugt HA, Kranse R, Steyerberg EW, van der Poel HG, Busstra M, et al. Prospective validation of a risk calculator which calculates the probability of a positive prostate biopsy in a contemporary clinical cohort. Eur J Cancer. 48(12): 1809-15 (2012).
52. Nguyen CT, Yu C, Moussa A, Kattan MW, Jones JS, et al. Performance of prostate cancer prevention trial risk calculator in a contemporary cohort screened for prostate cancer and diagnosed by extended prostate biopsy. J Urol. 183(2): 529-33 (2010).
53. Moyer VA. U.S. Preventive Services Task Force. Screening for prostate cancer: U.S. preventive services task force recommendation statement. Ann Intern Med. 157(2): 120-34 (2012).
54. Daskivich TJ, Lai J, Dick AW, Setodji CM, Hanley JM, et al. Urologic diseases in America project. Variation in treatment associated with life expectancy in a population-based cohort of men with early-stage prostate cancer. Cancer. 120(23): 3642-50 (2014).
55. Newschaffer CJ, Otani K, McDonald MK, Penberthy LT. Causes of death in elderly prostate cancer patients and in a comparison non-prostate cancer cohort. J Natl Cancer Inst. 92(8): 613-21 (2000).
56. Briganti A, Spahn M, Joniau S, Gontero P, Bianchi M, et al. European Multicenter Prostate Cancer Clinical and Translational Research Group (EMPaCT). Impact of age and comorbidities on long-term survival of patients with high-risk prostate cancer treated with radical prostatectomy: A multi-institutional competing-risks analysis. Eur Urol. 63(4): 693-701 (2013).
57. MacKintosh FR, Sprenkle PC, Walter LC, Rawson L, Karnes RJ, et al. Age and prostate-specific antigen level prior to diagnosis predict risk of death from prostate cancer. Front Oncol. 6: 157 (2016).
58. Cooperberg MR, Lubeck DP, Meng MV, Mehta SS, Carroll PR. The changing face of low-risk prostate cancer: Trends in clinical presentation and primary management. J Clin Oncol. 22(11): 2141-2149 (2004).
59. Schwartz KL, Alibhai SM, Tomlinson G, Naglie G, Krahn MD. Continued undertreatment of older men with localized prostate cancer. Urology. 62(5): 860-865 (2003).
60. Townsley C, Pond GR, Peloza B, Kok J, Naidoo K, et al. Analysis of treatment practices for elderly cancer patients in Ontario, Canada. J Clin Oncol. 23(16): 3802-3810 (2005).
61. Konety BR, Cowan JE, Carroll PR. Patterns of primary and secondary therapy for prostate cancer in elderly men: Analysis of data from CaPSURE. J Urol. 179(5): 1797-1803 (2008).
62. Richstone L, Bianco FJ, Shah HH, Kattan MW, Eastham JA, et al. Radical prostatectomy in men aged ≥ 70 years: Effect of age on upgrading, upstaging, and the accuracy of a preoperative nomogram. BJU Int. 101(5): 541-6 (2008).
63. Greco KA, Meeks JJ, Wu S, Nadler RB. Robot-assisted radical prostatectomy in men aged ≥ years. BJU Int. 104(10): 1492-5 (2009).
64. Bechis SK, Carroll PR, Cooperberg MR. Impact of age at diagnosis on prostate cancer treatment and survival. J Clin Oncol. 29(2): 235-41 (2011).
65. Lunardi P, Ploussard G, Grosclaude P, Roumiguié M, Soulié M, et al. Current impact of age and comorbidity assessment on prostate cancer treatment choice and over/undertreatment risk. World J Urol. 35(4): 587-593 (2017).